Meet Alevia Pharma: a startup developing OraSpray for the local management of pain due to mouth ulcers caused by cancer therapy (called oral mucositis). This severe pain impacts nutrition, speech, compliance with cancer therapy, and quality of life, and is currently treated with systemic opioids.
CCEI’s Kate Savinelli was able to sit down with CEO Sam Nanayakkara and COO Sophie Takmopoulos to discuss Alevia Pharma in more detail.
—
In the realm of innovative healthcare solutions, Alevia Pharma stands out as a beacon of hope for cancer patients suffering from oral mucositis — a common and painful side effect of chemotherapy and radiation therapy. This promising startup is developing an oral spray designed to alleviate the debilitating symptoms of this condition, enabling patients to regain their quality of life.
The inception of Alevia Pharma can be traced back to Dr. Rajesh V. Lalla, D.D.S., Ph.D., a research professor at UConn’s School of Dental Medicine. Dr. Lalla, with his extensive background in the field, identified a critical need for effective solutions for oral mucositis patients.
His research led to key discoveries that laid the groundwork for Alevia Pharma. The company officially took shape when CEO Sam Nanayakkara, an experienced entrepreneur and faculty member in the UConn School of Business, met Dr. Lalla at the Connecticut Innovation Invention Center.
“Rajesh had this idea and wanted to do something with his invention. I wanted to make a purposeful impact, and this was a perfect fit,” Nanayakkara recalled.
Joining Nanayakkara and Dr. Lalla is COO Sophie Takmopoulos, a former student of Nanayakkara’s course on entrepreneurship and technology innovation.
Takmopoulos, enthusiastic about the project, said, “I was immediately drawn to the potential impact of the product. It’s been a great journey since.”
The team also includes Dr. Diane J. Burgess, a research professor at the UConn School of Pharmacy, who contributes to the formulation aspect of the product.
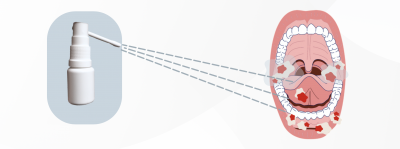
Alevia Pharma’s flagship product is an oral spray that transforms into a gel upon application, adhering to the mouth’s inner surface. This gel delivers a non-addictive local anesthetic, providing much-needed pain relief for patients with oral mucositis.
“Patients with oral mucositis often struggle with eating, drinking, and speaking, which can lead to severe complications,” Takmopoulos explained. “Our product offers a non-opioid solution, which is a significant step forward considering the current opioid crisis.”
The vision of Alevia Pharma extends beyond a single product.
“We are building a whole platform. Our delivery mechanism can be adapted for various drug agents — not just for cancer-related conditions but potentially for other medical needs,” Nanayakkara emphasized.
This broader approach reflects the company’s commitment to developing a portfolio of innovative solutions for those suffering from severe medical conditions.
The journey of Alevia Pharma has not been without its challenges. One of the major obstacles was developing a cohesive brand and team dynamic.
“Coming up with the company name and our branding took a lot of time and effort,” Takmopoulos noted. “But it was essential for aligning our mission with our identity.”
Despite these challenges, the company has achieved significant milestones. Recently, Alevia Pharma secured approval for a Phase 0 proof of concept study, allowing them to test their product on human subjects. This achievement marks a crucial step towards clinical trials and potential FDA approval.
“It’s a big success for us, as it means we can demonstrate our product’s effectiveness in a controlled setting,” said Nanayakkara.
Alevia Pharma has actively participated in various accelerator programs and pitch competitions, including CCEI’s Accelerate UConn program, and the Northeast NSF I-Corps program. These experiences have provided valuable insights and connections within the healthcare community.
“Engaging with patients and oncologists has been enlightening,” Takmopoulos said. “It’s heartwarming to see the trust and hope that our work inspires in the cancer community.”
Looking ahead, the company is strategically planning its next steps, including securing additional funding and navigating the FDA approval process. They are currently utilizing the $15,000 of non-dilutive funding from CCEI’s Summer Fellowship Accelerator to hire a regulatory consultant to facilitate this process.